AccuGenomics: Illegal Services Exposed (Update 2024)
AccuGenomics creates a special spike-in control technique that enhances the precision & effectiveness of clinical sequencing experiments.

Why AccuGenomics is in trouble?
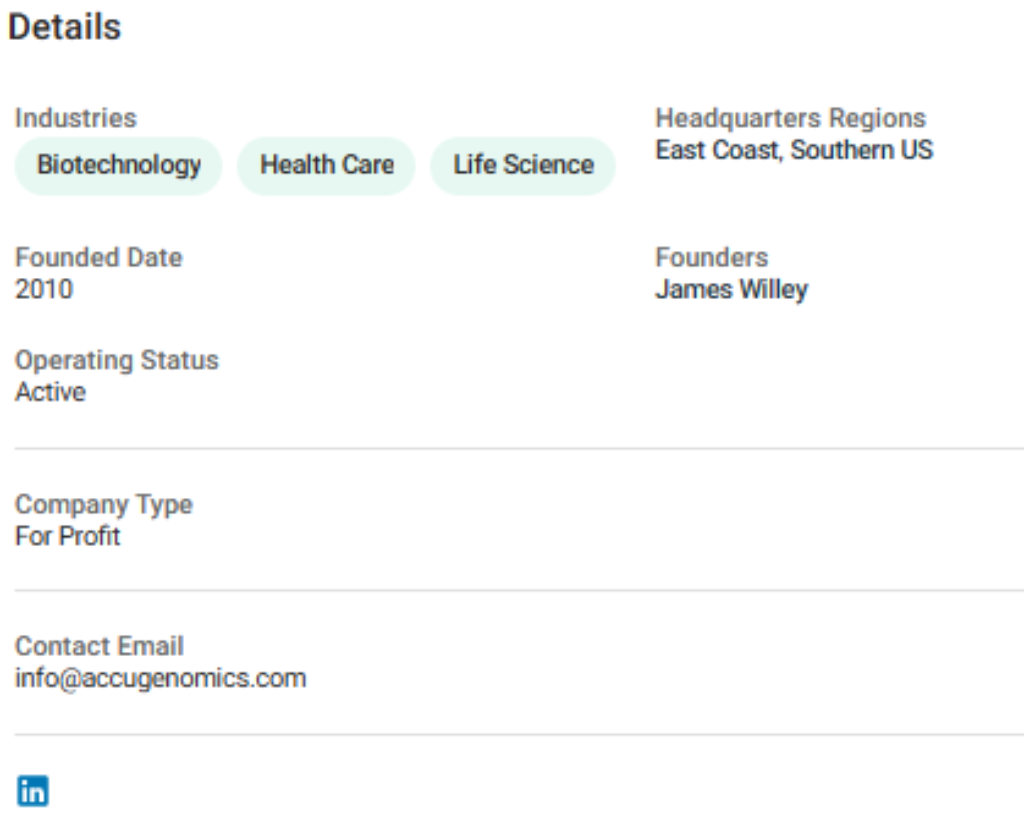
Ronald B. McNeill, Nick Lazaridis (President of AccuGenomics), John Anders, and Jerry Vardzel (“petitioning creditors”) filed an obligatory petition under the Bankruptcy Code on October 14, 2010
What is Bankruptcy?
Through the legal process of bankruptcy, individuals or other entities that are unable to pay their creditors back can seek partial or complete relief from their debts. Bankruptcy usually happens by a court order that is frequently requested by the debtor.
On November 11, 2012, the trustee filed a case in which, among other things, it was claimed that the institutions’ acts violated the automatic stay that was in place when Toledo filed a petition to dismiss on January 11, 2013. On January 11, 2013, Rochester formally filed its motion to dismiss.

On October 19, 2010, James Willey, a representative of Toledo, met with the management of Eman 2, LLC, the company that later became defendant Accugenomics, Inc. (“Accugenomics”), to discuss a potential private acquisition of the Toledo patent licenses.
According to Toledo, as of October 31, 2010, the debtor’s license rights to the patents were no longer valid. Toledo suspended all discussions with Accugenomics on these licenses on November 15, 2010, nevertheless, to better understand its stance.
A person or other entity that cannot pay their debts to creditors may seek discharge from some or all of their obligations through the legal process known as bankruptcy. Most commonly, the debtor initiates a court order requiring bankruptcy before it can be implemented.
Rochester purportedly terminated the debtor’s licenses as a result of the bankruptcy in a letter it issued to defendant Alfred C. Pollock, the debtor’s chairman of the board, on November 4, 2010.
Without taking any action to accept or reject the licenses with the universities, the 60-day window the trustee had to assume or reject an executory contract of the debtor ended on January 12, 2011. Rochester filed a request to ascertain the estate’s property on June 14, 2012, asking for a finding of the estate’s interest in specific intellectual property.

On July 9, 2012, the trustee formally declared his decision to relinquish the estate’s claims to the patents from Rochester and Toledo by filing notifications of abandonment. As a result of the rights being cumbersome and of little value to the estate, the land was abandoned.
Declared the notice –
The trustee declared in the notices that he was not giving up any claims [the debtor] might have against [the university], Accugenomics, the Petitioning Creditors, or any other party for violating the automatic stay, their participation in an effort to deny the estate access to the License Rights, or any other action that caused the License Rights to lose significance for the estate, or any other claims [the debtor] may have in this case.
The trustee claims in his complaint that Rochester and Toledo violated the automatic stay by relying on the cancellation of the debtor’s license rights as a result of the debtor’s bankruptcy and by negotiating to license those rights without the debtor’s consent with defendants Lazaridis, McNeill, Eman 2, LLC, and Accugenomics. According to the trustee, the plaintiff suffered damages from the institutions and other identified defendants totaling more than $10,000.00 as a result of their actions
About AccuGenomics
For superior outcomes for patients, AccuGenomics’ objective is to increase the precision and effectiveness of clinical sequencing testing. The requirement to show the analytical performance of Companion Diagnostic (CDx) tests to patients and healthcare professionals is growing as the speed of innovation in NGS diagnostics keeps expanding the boundaries of detection for negligible-abundance biomarkers. To eliminate false positive and false negative findings and reveal the real fundamental biology, we develop internal standards for targeted NGS tests. These standards help physicians and patients make educated healthcare choices while reducing ambiguity.
The highest performance levels TM of their sequencing assays may be shown by test creators and those who implement thanks to technology AccuGenomics has created and made available for purchase. A variety of very sensitive NGS applications, such as quick accidental agent identification in cell therapy manufacturing and variant detection in liquid biopsies, are made possible by SNAQ-SEQ (Standardized Nucleic Acid Quantification for Sequencing).
AccuGenomics is company President Nick Lazaridis, Ph.D.

Nick, shared additional details about the products and services offered by AccuGenomics.
They claim that they create internal spikes for standards that are manufactured so that low abundance biomarkers from liquid biopsy or other sample types may be accurately detected (can be any target, any system, and any sample type). They also claim to raise the real limit of detection (LOD) for NGS assays such that even uncommon variations may be recognized, allowing for faster identification of cancer molecular markers even from liquid biopsy samples. About NGS testing, these internal guidelines offer advancements in a variety of areas:
- enhance accuracy and make it possible to assess actual LOD, which improves patient outcomes.
- are created to be biochemically similar to the target or variation of interest; Internal standards reduce false positives and false negatives, enhancing the precision of all targeted NGS test panels.
- during sample extraction, are co-processed and co-varied with the target of interest, and are spiked into each sample at known concentrations. This creates the ideal QC workflow.
- offers pathologists gold-standard reporting as copies of variant/ml of plasma
AccuGenomics claims that they are a revolutionary accuracy diagnostics technology firm that develops tools (assay-specific bespoke internal standards) to accurately measure biology, enabling molecular diagnostic procedures to be even across platforms and dispersed testing locations, more precise, standardized, and harmonized.
They also claim order to accurately call additional true positives with low abundance (which can be incorrectly called false negatives if using pre-defined threshold algorithms provided by Dx test makers), it is crucial to be able to increase sensitivity without sacrificing specificity in NGS testing and diagnosis. Internal synthetic standards that biochemically resemble the variation of interest can be used to get a direct orthogonal measurement to establish a target-specific LOD for every sample, thereby adjusting the detection limit to each one.
Along with the reduction of false positives and false negatives that would otherwise result in inaccurate variant calls and patient therapies, they claim that their researchers have demonstrated an increase of 3-5-fold better sensitivity with targeted NGS panels. The usage of an internal spike in standards has a distinct favorable effect on precision medicine initiatives, and several of their pharma partners are recognizing the technology’s significant utility for determining transcripts’ quantity.
Conclusion
Therefore, the trustee’s claims for relief against Rochester and Toledo for breaches of the automatic stay have a reasonable basis in the facts as detailed in his complaint. In light of this, Rochester’s and Toledo’s petitions to dismiss for failure to allege a claim on which relief may be given are DENIED.







