Reading the story’s title must have aroused your interest in discovering more about this terrible guy. As the former Director of Gynecology at Aurora Healthcare in Milwaukee, Dr. Scott Kamelle is a well-known gynecologic oncologist and claims to be reputable in his working field.
He graduated from the University of California, Berkeley with a Bachelor of Science in Chemistry and a Minor in Dramatic Art. He then attended the Boston University School of Medicine for his medical degree and completed an Obstetrics and Gynecology residency at the Hospital of the University of Pennsylvania.
The University of Oklahoma Health Sciences Center’s fellowship allowed Dr. Scott Kamelle to extend his training in gynecologic oncology. But hold on, despite all of these advantages, he still engaged in unlawful conduct that led to legal action. So come along with me as I immerse you in the Dr. Scott Kamelle narrative.
Allegations against Dr. Scott Kamelle
The Aurora St. Luke’s Medical Center in Milwaukee is home to gynecologic oncologist Scott Kamelle, MD. Numerous grievances and legal actions about his conduct as a professional led to increased scrutiny of him.
The death of a patient who bled out has Dr. Scott Kamelle under fire. The patient’s husband, Bill Adamson, said in a letter to Aurora’s chief medical officer that Dr. Scott Kamelle lied about seeing his wife and took hours to return phone calls.
Blood clots were found in Ms. Adamson’s drainage bulb on July 16, 2016, which was discovered by the pair. Following the directions, they called the office. However, they were informed that Scott Kamelle was on call and would call them back shortly. He allegedly never followed through, and Ms. Rita Adamson died at home.

The Wisconsin Department of Safety and Professional Services, which issues physician licenses, was informed of the occurrence by Mr. Adamson, but no laws or regulations were broken. Dr. Scott Kamelle was not found at fault by Aurora either. A spokesman for the hospital said to PBS that the “on-call procedure that day failed to operate as anticipated and has been rectified.”
There was no reprimand given. But issues have come up again in a DSPS complaint from a doctor who came forward with information and filed a federal claim in 2020 that was later abandoned after the government declined to participate as a plaintiff.

Concerns about Dr. Kamelle’s methods are raised in the whistleblower lawsuit and by seven doctors who were questioned by the magazine.
The claims include that Dr. Kamelle commonly inserted powder and mesh devices, implanted ureteral stents, and included additional surgeons in the majority of surgeries without a need for them, which resulted in higher costs and probable risks.
In a report on one of the doctor’s whistleblower statements, a DSPS investigator stated, “The impression at Aurora is that Dr. Scott Kamelle is untouchable.” The “administration will not address concerns about Dr. Scott Kamelle that have been raised to their knowledge.”

According to the DSPS complaint, Dr. Kamelle’s robotic procedures cost twice as much, had “substantially greater incidences of pelvic infections,” required longer hospital stays, and had higher open surgery rates than those of peers.
The claims were denied by Aurora. Frank LaVora, MD, chief medical officer for Aurora’s Greater Milwaukee patient care region, stated to PBS that “the claims were carefully reviewed through an effective procedure that included an independent physician analysis.”
An investigation, according to him, found that Dr. Kamelle “continuously fulfilled every criterion of care and no chances for clinical enhancement were identified.”
Dr. Kamelle is accused of routinely implanting unneeded meshes and biologic powder, according to a third federal false claims case filed by a former Aurora doctor. This behavior is said to have been motivated by Dr. Kamelle’s claimed personal friendship with an ACell sales representative, a company that makes medical devices.
Dr. Scott Kamelle: Some Other Allegations
The whistleblowing doctor who brought the federal lawsuit raised concerns about Kamelle’s practice again in a DSPS complaint.
Through an open documents request, Wisconsin Watch was able to obtain the DSPS complaint and persuade a magistrate court to unseal the complaint that was filed in the U.S. District Court for the Eastern District of Wisconsin.
After the government decided not to join the action as a plaintiff, the informant dropped the complaint in 2020. Although the lawsuit was opened, the court denied releasing any documents that contained “confidential data about the status of the government’s inquiry at that moment as well as the investigatory techniques employed by the governments.”
The doctor asked to remain anonymous, along with the other seven doctors Wisconsin Watch spoke with, out of concern about possible professional retaliation for their statements. A number of the whistleblower’s charges were supported by the seven doctors as they collectively recalled their own experiences with Kamelle’s alleged actions.
Furthermore, studies reveal that one of Kamelle’s procedures, regular ureteral stent implantation, is infrequently warranted and happens in less than 3% of significant inpatient gynecological operations.
According to emails, the doctor raised concerns with hospital officials. Wisconsin Watch was informed by two more doctors that they had also asked hospital administrators for help.
One of the doctors interviewed for the case stated, “The perception at Aurora is that Dr. Kamelle is untouchable,” according to a DSPS investigator’s document summarizing the statement. The doctor stated, “Leadership was unwilling to tackle problems raised to their awareness involving Dr. Scott Kamelle.”
Dr. Scott Kamelle: Filled lawsuits for false claims
Individual whistleblowers, known as “relators,” can file civil or criminal accusations against persons who have deceived the federal government using such legal procedures, also known as qui tam cases. Instances of Medicare and Medicaid fraud are frequently found and prosecuted using this method.
You run the risk of jeopardizing your medical care, wasting tax resources, and breaking the law if you purposefully misrepresent others to obtain unauthorized benefits. Up to ten years in prison and fines of up to $500,000 are the consequences for enabling a Medicaid service or number to be used improperly.
Three years prior, a former employee of ACell filed his qui tam lawsuit in Maryland against the business, alleging, among other things, that the company had falsely marketed one of its goods, a “powder wound-dressing” called MicroMatrix, for internal use when the Food and Drug Administration had only given it approval for use externally.
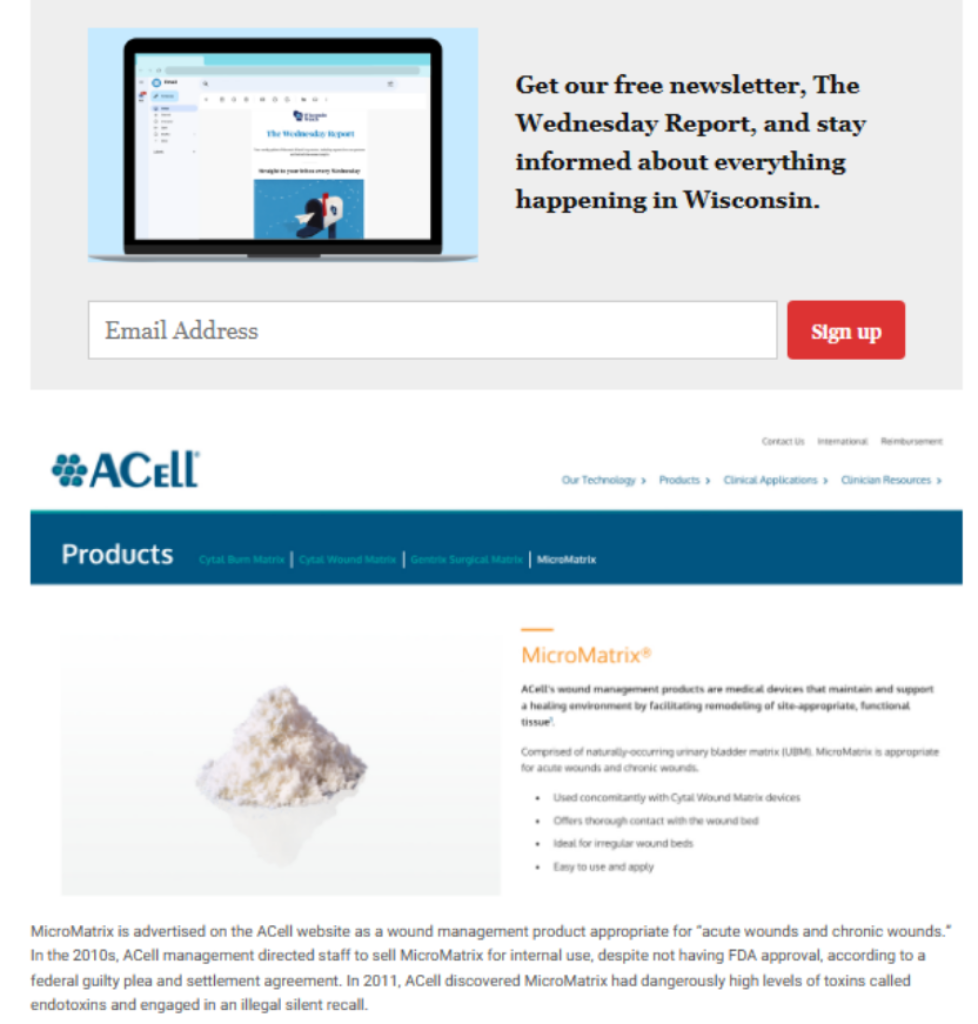
A civil resolution from ACell was reached in 2019 as a result of the Maryland case. According to a press release from the U.S. Department of Justice, the firm consented to pay $15 million to resolve criminal and civil liabilities stemming from claims that, in part, ACell “caused false claims to be filed to federal health care programs” for MicroMatrix.
In addition, ACell admitted guilt to one violation for neglecting and refusing to notify the FDA of a product recall. The False Claims Act enables whistleblower relators to recoup a part of any money that was falsely billed. Nearly $2.4 million was given to the former ACell worker.
Dr. Scott Kamelle: Utilization of contaminated Products in his surgeries
According to the federal guilty plea and settlement agreement, ACell’s “senior executives instructed its marketing staff to sell MicroMatrix for internal uses” despite the lack of FDA approval.
In addition, some of the substance that ACell improperly urged doctors to use internally was tainted. In 2011, the business found that MicroMatrix contained endotoxins in dangerously high concentrations.

Fever, infection, septic shock, and death are all possible effects of endotoxin exposure. For a medical device designed for internal implantation, the FDA establishes a limit of 20 units for the allowable level of endotoxins. Some had as many as 272 units, according to ACell.
PSMX Sheets, a different product that was authorized for internal use, also contained dangerously high levels of contamination. When ACell issued a recall, it also informed the FDA and the general public.
The plea agreement states, however, that ACell “took no action to reactivate or remove” the infected MicroMatrix.
After an additional round of tests in January 2012 revealed dangerously high amounts of endotoxins in MicroMatrix, the business started a stealthy, selective recall.
Despite knowing that the bigger packages of tainted MicroMatrix “were used internally” — once more, in violation of FDA standards — the company’s senior management took them out of circulation quietly, under the plea agreement, but it failed to alert the FDA.
In addition, ACell failed to inform its sales team, hospitals, or medical professionals about the contamination. Even after being told to return the infected, larger parcels to the corporate office, some salespeople continued to unintentionally sell them.
Considering that the contamination in the smaller batches of MicroMatrix carried a similar degree of risk, ACell took no action. Due to the contamination’s “too much street value,” according to the CEO of the company, it was not removed from the smaller containers, nor was it disclosed to physicians or hospitals.
When several medical specialists informed ACell that their patients experienced issues, the business once more failed to reveal the contamination.
Dr. Scott Kamelle frequently employed ACell
As I previously mentioned, Dr. Scott Kamelle was charged with “routine introducing of unneeded meshes and biologic powder” in his continuing DSPS complaint by the doctor who filed it. This is allegedly because of their close connection.
According to DSPS, a second doctor who asked Wisconsin Watch not to identify him testified regarding Kamelle’s use of ACell mesh while under oath to the U.S. Attorney’s Office.
The doctor fiercely defended the whistleblower, calling him a “very good doctor” whom he would trust to operate on his wife, according to a report of that DSPS interview from January 2022 that was done as part of Kamelle’s unsuccessful case against the whistleblower.
The agency paper stated that he had a different opinion of Kamelle and asserted that the ACell agent had been the best man at Kamelle’s wedding.
A close bond between Dr. Scott Kamelle and the ACell representative could be seen, according to two other witnesses to Kamelle’s surgical procedures who requested anonymity to safeguard their jobs. One of the witnesses recalled the ACell person as being there at the hospital “all the time.”
According to openly available records, Scott Kamelle claimed that ACell provided her with $8,135 in non-accredited training, meals, and refreshments between 2013 and 2016. In contrast to his Aurora colleagues, two gynecologic oncologists only earned $100 and $160 in food and drink from ACell. One received no payments from ACell.
Throughout a single day, ACell paid Dr. Scott Kamelle for food and drinks totaling up to $231. “Aurora caregivers may accept a modest meal” of no more than $25 for breakfast and lunch and $50 for dinner, according to a 2010–2016 modification to an Aurora policy on gifts and business courtesy.
Utilization of Mesh by Dr. Scott Kamelle
The whistleblower claimed that Dr. Scott Kamelle frequently defended the use of the mesh to avoid vaginal cuff dehiscence, which occurs when the top of the vagina tears. This story was supported by a second doctor who declined to give his name out of concern for retaliation.
While mesh has been utilized to alleviate severe cases of this problem, studies reveal that it is extremely uncommon, occurring in much less than 1% of hysterectomies.
Given that ACell voluntarily recalled their tainted PSMX mesh sheets in 2011, endotoxin contamination is less of an issue here.
However, according to the DSPS complaint, Kamelle had “substantially higher rates of vaginal infections, longer hospital stays, and higher rates of open surgery” in comparison to two other Aurora gynecologic oncologists. This was discovered during an internal evaluation of complications from gynecological surgeries from 2013 to 2015.
The complaint claims that the findings led to a review of Kamelle’s surgical practice by providers, which was later “discontinued by Aurora administration.”
Between 2011 and 2016, four more individuals who witnessed Kamelle’s operations gave Wisconsin Watch their official confirmation that they had seen her regularly employing ACell material. To save their professional reputations, they all requested anonymity. These two witnesses remembered Kamelle utilizing the powdered MicroMatrix, which was not permitted for internal usage.
The complaint asserts that he thinks Kamelle habitually utilized the mesh and MicroMatrix powder without alerting or getting permission from his patients. He asserts that he interacted with patients in the surgeries he took over from Kamelle, as well as an ACell representative and other staff members, and that he reviewed surgical consent documents.
Use of stents by Dr. Scott Kamelle
The complainant further asserts that Dr. Scott Kamelle performed almost all major procedures using ureteral stents, which he asserted was medically unnecessary and could result in difficulties.
The ureters, tubes that convey urine from the kidneys to the bladder, are kept open by stents. The installation of a stent increases the risk of infection, which may result in kidney failure, as well as blood or clots in the urine. Unnecessary stenting might cause difficulties and increase time and money.

Conclusion
After reading the entire report, I concluded that the circumstances surrounding Rita Adamson’s medical care and Dr. Scott Kamelle’s behavior raise major questions about the standard of care as well as accountability in the healthcare system.
Rita’s terrible death while under Dr. Kamelle’s care, as well as claims of unprofessional conduct and pointless operations, highlight the demand for a comprehensive and unbiased examination of the situation.
It suggests a possible culture of silence and protection surrounding Dr. Kamelle’s actions that other doctors have come forward as whistleblowers out of fear of professional retaliation.
The Wisconsin Department of Safety and Professional Services (DSPS), despite the complaints and supporting documentation, did not discover any legal or procedural violations, and Aurora St. Luke’s Medical Center did not hold Dr. Kamelle liable for his conduct.
The perception that he is untouchable may increase as a result of this inaction, which also casts doubt on the efficacy of the current supervision procedures.
The matter is made much more complicated by the False Claims Act and qui tam lawsuits. Although such legal measures can help identify and prosecute incidents of healthcare fraud, the results of the inquiry against Dr. Kamelle did not result in supporting the charges.
In the end, Rita Adamson’s case and Dr. Kamelle’s deeds necessitate a thorough examination of the protocols, safeguards, and dedication to the openness of the medical system.
It emphasizes how crucial it is to protect patient safety and hold medical staff accountable for their conduct to avoid future tragedies of this nature.










Doctors like Scott Kamelle always care about money and they overcharge the patients and do unnecessary procedures which is insane. How can anyone do these types of surgeries on their clients why they don’t think that these types of procedures can damage the internal parts of the patients?
How is this monster still alive in society, he has committed numerous medical frauds and false bill representation, and for these types of acts, the higher authority may take strict action on these types of issues.
It is the surgeons’ job to care for their patients, as they are paying for the services. However, in the instance of Dr. Scott Kamelle, they were liable to their patients, and the patients’ experiences show how inadequate the shameless creature he is.
These types of medical fraud and lawsuits are not allowed, and these types of issues can’t be solved. The doctors who have done this should be behind bars, and in court, it was concluded that he was using products that are not used for internal use, using these products without the permission of the higher authority, is unacceptable.